Research Highlights
Turn Me Off, Please!
Institutions:
Guangzhou Medical University, China
Team:
Yang, F.M., Shen, L., Fan, D.D., Bai, Y., Li, B., and Lee, J.
Disease Model:
Inflammation
Hydrogel:
VitroGel® 3D
Cells grown in 3D cell culture reveal how the inflammation pathway works, and what possible new protein variants can be designed to suppress TNF-induced inflammation.
Inflammation, be it chronic or acute, often results from a mis-regulation of natural immune functions. On the other hand, there exist elements in the immune system whose role is to maintain homeostasis. These elements may be tapped for therapeutic anti-inflammatory properties if their mechanisms of action could be properly elucidated. One such element is the regulatory protein YAP (yes-associated protein). In this report, the authors sought to understand how various isoforms (similar proteins formed by alternative splicing) of YAP could exert modulation on other proteins and thus temper the inflammation that can be induced in the cell.
A group of cell biologists from Guangzhou Medical University took on this challenge. They examined the cellular events that are involved in TNF-induced gene expression in both 2D and 3D cell culture models. Tumor necrosis factor (TNF) is a cytokine, a molecule that plays a key role in cell signaling during the immune response. Thus, TNF is potentially in the same sphere of influence as YAP, because YAP is known to be a transcriptional activator, at least in the presence of other proteins.
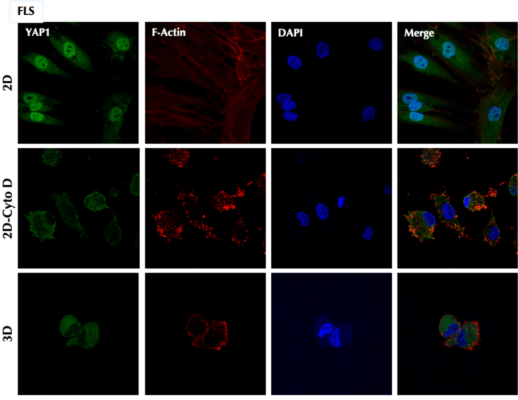
The authors used TheWell Biosciences’ VitroGel® 3D for their three-dimensional cell culturing. They used fibroblast-like synoviocytes (FLS) as their target cells, because these are known to be integrally involved in the inflammation-intense diseases, especially rheumatoid arthritis. To test cellular responses to various protein additives, they measured variations in FLS characteristics either in traditional plastic culture plates (2D) or in the hydrogel matrix provided by VitroGel 3D. They examined cellular changes as a function of added proteins by various means, including ELISA, total RNA sequencing (RNAseq) and quantitative PCR (qPCR). Also, they determined where the YAP isoforms and other proteins were expressed by using fluorescence and confocal microscopy. Typically, the authors cultured the cells in VitroGel 3D for seven days prior to stimulation with TNF.
The team found that YAP1 isoform 9 (YAP9) suppressed the cellular responses to TNF, while isoform 2 (YAP2) enhanced the responses. They determined that the YAP is mostly localized in the cytosol of FLS cells cultured in 3D medium. Notably, this was a different result from that shown in 2D medium, such that the value of 3D cell culture in producing the most realistic biological conditions could be verified. It turns out that YAP9 must interact with another regulatory protein in the inflammatory pathway, called A20, to suppress inflammatory signaling. YAP9 binds to its targets via a short stretch of amino acids in a proline-rich domain. It is even possible to use short peptides to mimic the action of YAP9, suggesting that there may be the possibility of using smaller molecules as drugs against acute or chronic inflammation. This is important because a high percentage of patients treated for inflammation do not respond well to traditional TNF antagonists, and the knowledge gained in this study could lead to an alternative form of therapy.
Read the publication:
Related Products:


