Research Highlights
Hydrogels and Light – A powerful Anticancer Duo
Institutions:
Shanghai Key Laboratory of Regulatory Biology and Tongji University, China
Team:
Yu, Y., Wu, X., Wang, M., Liu, W., Zhang, L., Zhang, Y., Hu, Z., Zhou, X., Jiang, Q., Cai, F., and Ye, H.
Disease Model:
Melanoma
Hydrogel:
VitroGel® 3D
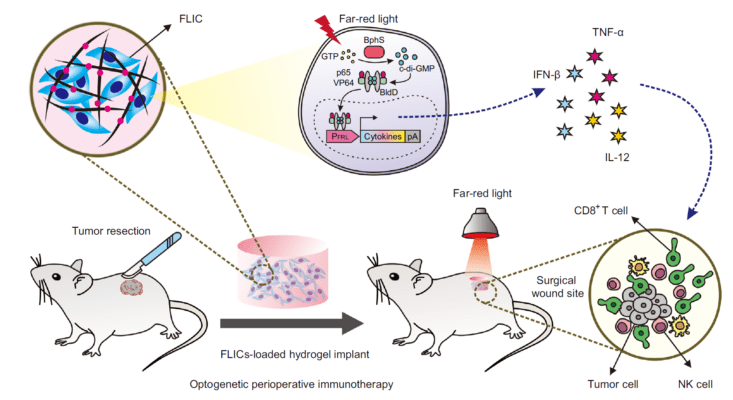
Engineered light-activated cytokine delivering cells can be encapsulated in VitroGel hydrogel for more efficient delivery to tissues to prevent tumor re-growth.
Light controlled drug delivery is an exciting biomedical frontier. Techniques such as photodynamic therapy (PDT) allow therapeutic agents to be activated only when irradiated by a specific wavelength of light, and only when at or near the target tissue in the body. Now this type of approach is being considered as a means to help traditional surgery, such as the removal of cancerous tumors. At times, even when tumor resection is achieved, there can be a regression and return of cancerous cells because of inadvertent triggering of undesirable immune responses. In this publication, the authors explore the use of engineered cells that can release their payloads upon irradiation with far-red light.
A team of cell biologists from China have created far-red light-controlled immunomodulatory engineered cells (FLICs) that can bring cytokines to their targets and release them when stimulated by light. They call this approach “optogenetic therapy.” The goal is to use it to treat solid tumors by promoting a desirable immune response by the host at the site of the tumor with a lowered chance of tumor re-growth.
Here, the authors advanced the science of immunotherapy, which exploits the body’s own natural killer (NK) cells and cytotoxic T lymphocytes to eat away at a tumor. They engineered human mesenchymal stem cells (MSCs) to contain bacterial-derived genes that can be triggered by the application of 730 nm red light, which can penetrate deeply into tissues. The ultimate event upon illumination with this light is the release of various cytokines; these molecules signal the stimulation of that wing of body’s immune response that can fight tumors. Importantly, the authors had to prevent the body from attacking these artificial cells before they reached their targets. To do so, they encased the FLICs in a hydrogel. Specifically, they mixed about 2.5 million FLIC cells with 240 μL of TheWell Bioscience’s VitroGel 3D hydrogel solution to create an implantable concoction. This was cultured in plastic plate in the lab and then applied to the wounds created in mice after the surgical removal of melanoma tumors.
The results were impressive. After allowing light-activated products from the implantable hydrogel to diffuse in the mouse bodies for several days, they examined tissues for the presence of cytokines and for the later recurrence of the skin cancer. Cytokine and NK cell levels were raised while local tumor recurrence was significantly inhibited by the FLIC-loaded hydrogel implants, compared to controls. The authors were able to demonstrate that these effects were indeed a result of the host’s T-cell activation. In the end, this study brings a new and powerful tool to the fight against cancer, and it shows once again that hydrogels have enormous utility as a biocompatible cell delivery agent.
Read the publication:
Related Products:


