Research Highlights
VitroGel Hydrogel as a Nurturing Matrix for Healing the Uterus
Institutions:
Hospital of China Medical University and Key Laboratory of Reproductive Dysfunction Diseases and Fertility Remodeling, China
Team:
Hao, X., Zhang, S., Li, P., Huang, J., Yuan, Z., and Tan, J.
Disease Model:
Intrauterine adhesions
Hydrogel:
VitroGel® RGD
The VitroGel RDG hydrogel matrix can assist in delivering mesenchymal stem cells to treat intrauterine adhesions and prevent them from returning after therapy.
Intrauterine adhesions, or IUA, are bands of fibrous tissue that can grow after physical damage, abrasion, or scarring of the uterus. They can cause menstrual bleeding and infertility. These lesions are typically treated by surgical removal and hormone treatment, but recurrence is common, approaching 2/3 of cases. Thus, additional therapeutic strategies are being sought, such as providing a secondary drug (i.e., an adjuvant) along with hormones.
In the last decade, the application of mesenchymal stem cells (MSCs) has shown promise to help repair IUA lesions and prevent them from coming back. In this case, the MSCs can be grown from bone marrow, adipose tissue, umbilical cord tissue, or amniotic fluid. While the use of MSCs has helped in many cases, the procedure stands to be improved.
In this study, a collaborative team of scientists from China assessed how well RGD (arginine-glycine-asparagine) peptides can help during the delivery of MSCs to IUA lesions. These peptides assist the binding of cells to one another. They can be fashioned in a hydrogel matrix, such as TheWell Bioscience’s VitroGel RGD, a xeno-free injectable milieu that facilitates the delivery of exogenous cells to target tissues.
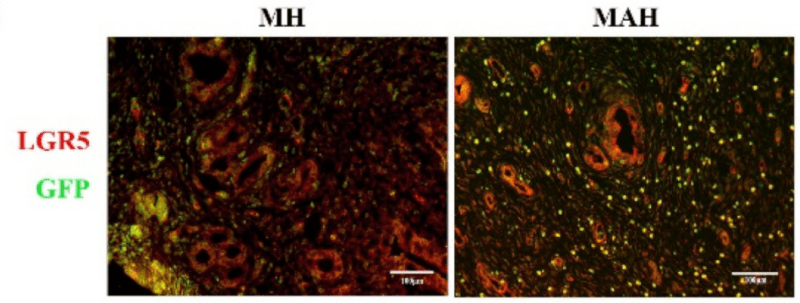
To test the capacity of RGD peptides to improve MSC-based therapy for IUA, the authors mixed MSCs with amniotic membrane extract (AME) and VitroGel RGD. They then employed this composite into a rat model for IUA to see if it could help repair uterine endometrial damage. To begin, they produced AME from fresh placentas obtained at the hospital. MSCs were then grown in this medium and then mixed with VitroGel RGD at a ratio of 1:4. Then they have injected rats with the hydrogel mix or a control mix after they had undergone simulated uterine scarring procedures. After about four estrus cycles, the rats were examined for IUA-like symptoms using bioluminescence staining, histology, and PCR. The authors found that the AME-enriched hydrogel was an excellent growth medium and highly biocompatible. They also found that this hydrogel improved the ability of the uterus to retain and grow MSCs. This occurred in IUA-like lesions, and the mix helped restore the uterine cavity’s shape. PCR tests showed that gene products related to inflammation were less in the hydrogel-treated tissues than in controls. Overall, the study demonstrated that the RGD hydrogel matrix could be a more promising means to deliver MSCs to IUA and promote the healing process, thereby lessening the probability of recurrence.
Read the publication:
Related Products: